Vinna við nýjar heimasíður Umhverfis- og orkustofnunar og Náttúruverndarstofnunar er í gangi. Heimasíða Umhverfisstofnunar er virk á meðan vinnunni stendur. Information in English
Label
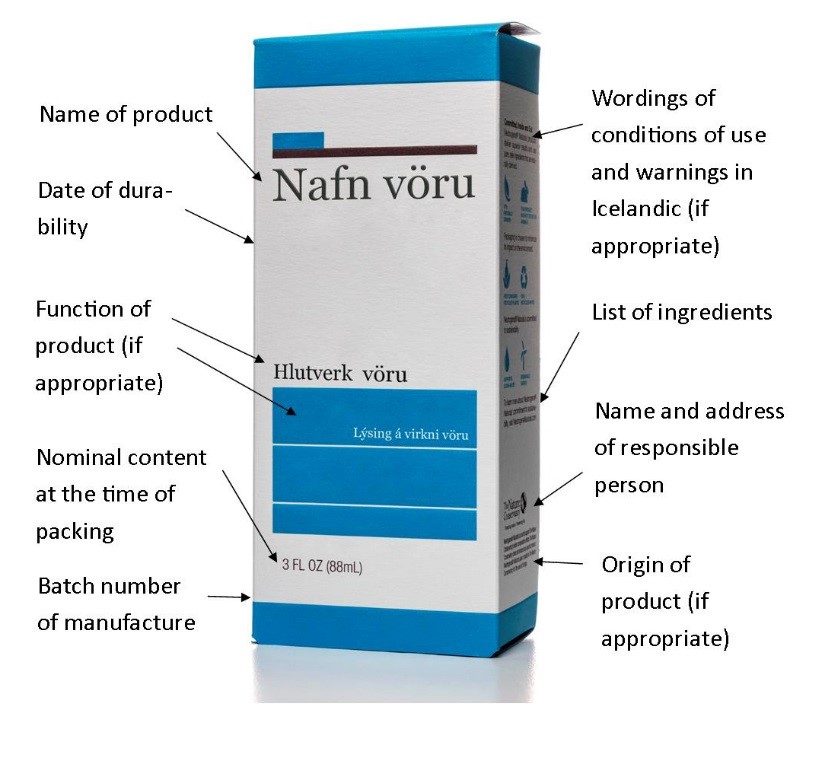
Labelling of cosmetic products shall be in Icelandic, English or Nordic language other than Finnish. However, certain labelling concerning conditions of use and warnings shall be in Icelandic. Following information shall be included on a label or a leaflet:
- Name of the cosmetic product.
- Function of the cosmetic product, unless it is clear from its presentation.
- Name and address of the responsible person.
- Country of origin of the cosmetic product if imported into the EEA.
- Nominal content at the time of packaging (weight or volume).
- Date of minimum durability.
- Wordings of conditions of use and warnings in Icelandic according to Icelandic translation of Annexes III.-VI. of the EC regulation of cosmetic products.
- Batch number of manufacture.
- List of ingredients (INCI name or other approved nomenclature). All nanomaterials shall be specified. Perfume and aromatics shall be referred to by the terms “perfume” or “aroma”.
See more information of labelling of cosmetic products in Article 19 of the EC Regulation of cosmetic products.